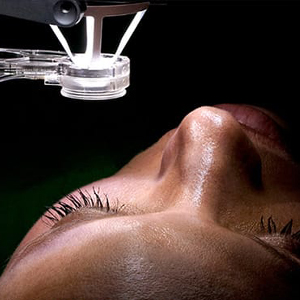
Human amniotic membrane has been shown to provide much benefit in the treatment of certain conjunctival and corneal conditions. Traditionally, amniotic membrane allografts have been transplanted with sutures or fibrin glue in the operating room. At present, the in-office use of sutureless allografts offer a patient-friendly, non-invasive, fast alternative with significant clinical outcomes. The acellular, precisely cut grafts act as a physical wound cover and barrier to protect the cornea, conjunctiva and epithelium as they heal. The graft will also reduce pain and irritation from friction by the eyelids on the ocular surface.
Amniotic membranes promote epithelial growth. They reduce cell death and inflammation and also possess antimicrobial properties that can decrease the rate of infections. The round and disc shaped grafts are available in a range of thicknesses and sizes for maximum versatility. In preparation and manufacture, the amniotic membrane allograft is subjected to a process of decellularization and stabilization that is followed by bathing in a low dose of gamma rays after which the membrane can be implanted and universally tolerated without a risk of rejection.